Continuous Inkjet Printers / Case Coder
Coding on Pharmaceutical and Medical Device Packaging
Strict global standards require clear lot and pharmaceutical date coding on all packaging. Companies need optimal solutions to ensure compliance and product safety. KEYENCE offers Continuous inkjet printers that monitor breakpoints for flawless dot quality, delivering consistent, reliable printing with a solvent-based ink system ideal for pharmaceutical and medical device packaging.
Barcode / 2D code
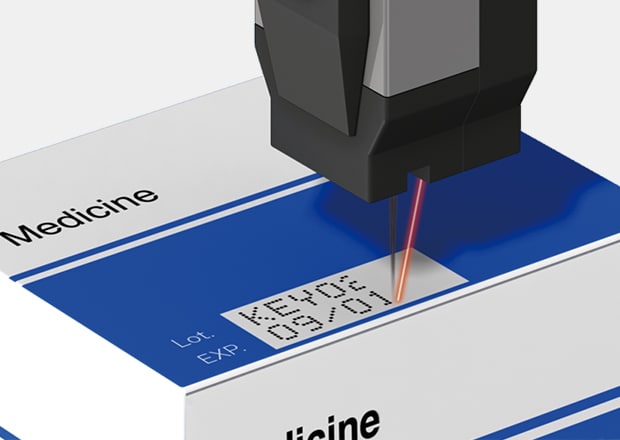
Installation example
Curious about our pricing?
Click here to find out more.
Date Coding and Marking on Pharmaceutical and Medical Device Packaging
CIJ printers enhance productivity, tracking, and traceability on various packaging materials. This includes:
- Packaging films, pouches, and labels
- Carboard boxes
- PET bottles
- Blister Packs
- Cartons
- Glass bottle and ampoules

Pouches

Cardboard boxed

PET bottles

Heat-sealed packages

Cartons

Glass containers
We’re here to provide you with more details.
Reach out today!
Coding and Marking Challenges in Pharmaceutical and Medical Device Industry Packaging
- Achieving Consistency Across Packaging Types
High-performance, adaptable coding technology for various packaging types offers seamless integration, minimal maintenance, product protection, and clear lot/date codes. - Meeting Regulatory Compliance
Adaptable, high-contrast, damage-free printing produces clear and compliant labeling on pharmaceutical and medical device packaging. - Ensuring Product Safety
Ensure product safety by providing consistent, high-quality, and legible prints for both OTC purchases and patient prescriptions. - Coding and Marking in High-Speed Production
KEYENCE CIJs offer easy integration and the ability to mark clear lot/date codes on a wide range of materials and shapes in even the most demanding production lines.
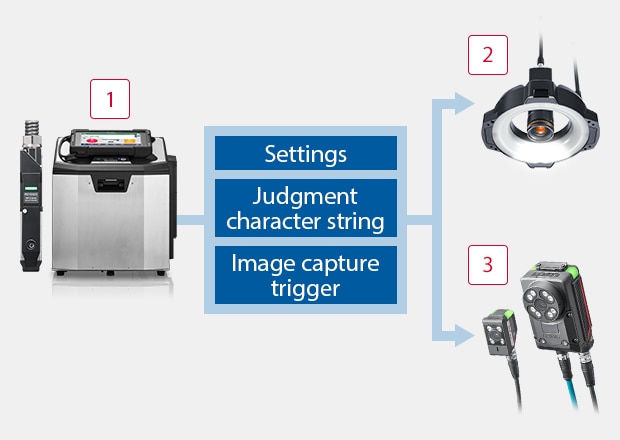
Easily Link to a Print Inspection Device with Just a LAN Cable
-
1Continuous Inkjet Printer MK-G Series
-
2Intuitive Vision System CV-X Series
-
3Vision Sensor with Built-in AI IV4 Series
Conventional Systems

Special safety equipment is necessary for maintenance
MK-G Series

Easy and safe maintenance for anyone
*Special safety equipment is reqired when performing cetain tasks such as replacing the pump.
Discover more about this product.
Click here to book your demo.
CIJ for Pharmaceutical and Medical Device Packaging
MK-G Series
The MK-G Series is a self-troubleshooting continuous inkjet printer featuring automation, such as hands-free cleaning, maintenance, and consistent performance. Its ability to self-diagnose significantly reduces the time needed to recover from errors without waiting for a technician.
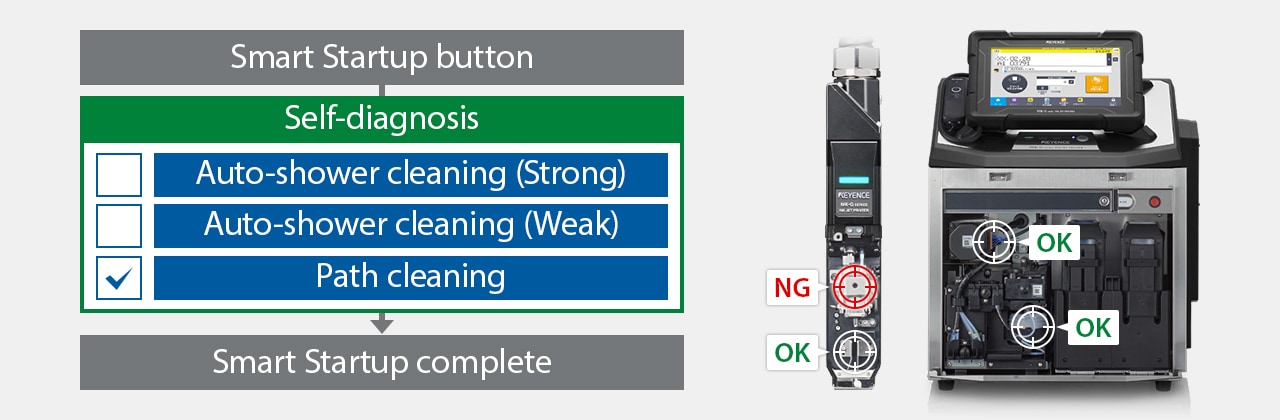
Using the MK Dock enables self-diagnosis and cleaning to ensure stable startup.
Get detailed information on our products by downloading our catalog.
View Catalog
FAQs about Coding, Serialization, and Marking on Packaging in the Pharmaceutical and Medical Device Industry
How Does the KEYENCE MK-G Series Marking and Coding Printer Specifically Address the Unique Needs of Coding on Pharmaceutical and Medical Device Packaging?
Promising a combination of benefits, MK-G Series printers can handle the wide range of coding requirements found in pharmaceutical and medical devices. Real-time quality checks, automated maintenance and diagnostics, and vast product compatibility handle many industry-specific needs. MK-G Series’ precise markings meet stringent formatting and content requirements set by regulatory bodies.
How Do Date Coding and Marking on Pharmaceutical and Medical Devices Contribute to Enhancing Anti-Counterfeiting Measures and Product Authentication?
This process ensures traceability through high-quality, reliable printed regulatory codes. Including expiration and manufacturing dates helps verify product authenticity, while maintaining high production throughput and efficiency.
What Are the Key Considerations When Implementing Marking and Coding Solutions onto Packaging Materials in the Pharmaceutical and Medical Devices Manufacturing Industries?
The inkjet coder should be able to seamlessly integrate into existing production lines. Throughput and marking speed are crucial to maintaining high production efficiency, while ease of maintenance and cleaning prevents downtime. Additionally, the solutions must comply with safety regulations to meet industry standards.
What Are the Main Challenges When Printing Date Codes on Pharmaceutical and Medical Device Packaging?
The main challenges include ensuring precise, durable codes that comply with regulations and serialization requirements. Maintaining high-speed production without compromising quality is also essential, and the ability to print consistently on various packaging materials adds to the complexity.
Related Downloads

This guide explains continuous inkjet (CIJ) printer applications together with pictures and illustrations. It contains many installation examples in various industries divided into food, medicine, and cosmetics; electrical machinery and electronics; and automotive, metal, and others. These examples show printing on targets specific to each industry.


![Process-specific Marking Applications [Food/Pharmaceutical Industry]](/img/asset/AS_133442_L.jpg)

